Imagine this scenario: a snorkeler or free diver approaches you and asks for a hit of your air — what do you do? Giving strangers compressed air may not actually be doing them a favor if you don’t know enough dive science to be safe. Earlier, we discussed the aspects of Charles’ Law and how it applies to SCUBA diving. This week we’ll look at the similar Boyle’s Law and how its principles relate to our favorite activity.
What is Boyle’s Law?
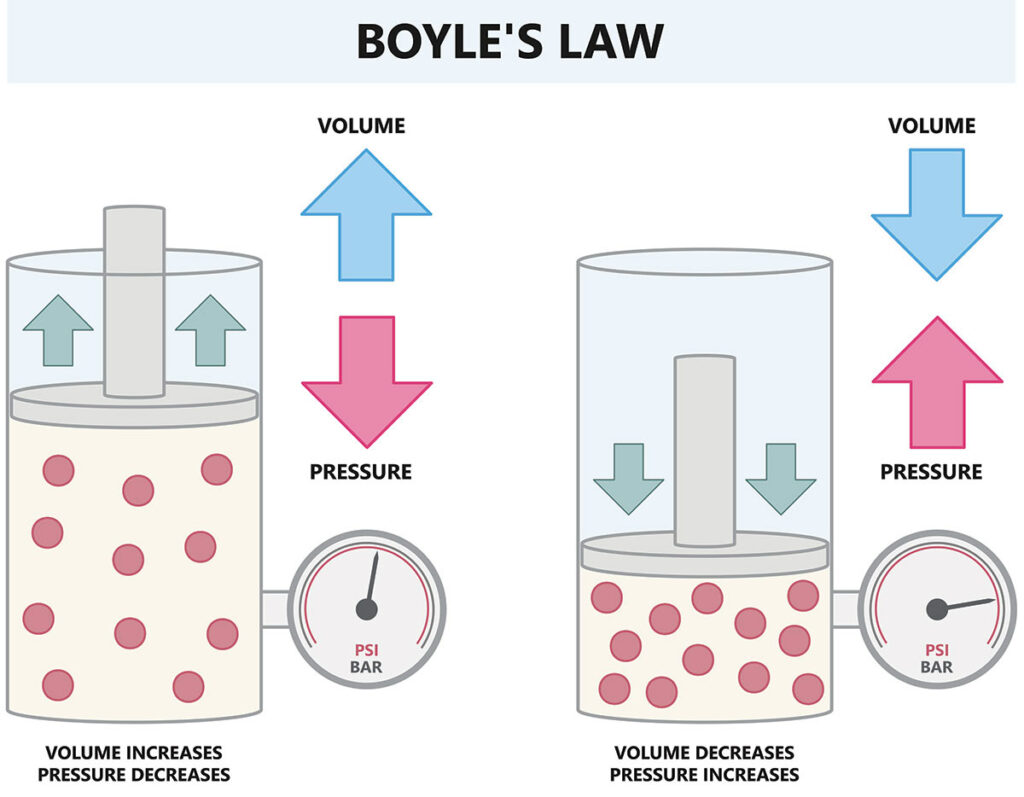
Robert Boyle was a chemist and a physicist who published this law in 1662. Boyle’s Law states that at constant temperature, pressure and volume are inversely proportional; as one increases, the other decreases. A balloon pushed underwater, for example, shrinks as its volume is compressed. Conversely, a balloon inflated underwater and released expands and may burst as it rises and its volume expands.
This principle is the foundation of SCUBA safety training to prevent barotrauma, or injury to the body from changing pressure. In simple English: If you increase pressure, the volume of the gas decreases, and if you increase the volume of the gas, the pressure decreases.
Here are some SCUBA diving rules related to Boyle’s Law.
Why is Boyle’s Law important to scuba diving?
Boyle’s law is extremely relevant to scuba diving. As a scuba diver descends underwater, the pressure on their body increases and the air spaces (lungs, mask, ears, sinuses) get compressed. As the scuba diver ascends, the pressure decreases and the air in the air spaces expands. There are two main ways that Boyle’s law is important for scuba diving:
Holding your breath
The number one rule of scuba diving is to never hold your breath. This is because holding your breath stops you from being able to equalize the air space in your lungs. If you were to ascend while holding your breath, then the air would expand and you could acquire serious injury to your lungs.
Ascending too fast
It is important to ascend slowly when scuba diving to avoid decompression sickness. While you scuba dive, your body takes on nitrogen from the air in the tank, ascending slowly gives the nitrogen time to off-gas safely. Ascending fast can cause the nitrogen to form bubbles, which expand and lead to decompression sickness.

Boyle’s Law & Scuba Diving Rules
Clear ears and descend slowly
Divers gently force air into their Eustachian tubes so that their eardrums do not burst inward from the vacuum created when the volume of air in the inner ear shrinks. They descend slowly, stopping as needed to adjust. Ascending too rapidly can cause the eardrum to burst outward if the air cannot escape back through the Eustachian tubes as it expands. Diving with a cold or allergy flare that swells the tubes and prevents clearing is ill-advised.
Breathe steadily
Controlled by the regulator, compressed air fills the lungs according to ambient pressure, allowing more oxygen in a breath as the lungs compress. Its volume expands or contracts with even a few feet of depth change, so Boyle’s Law means it’s important to breathe in and out without trying to save tank air by breath-holding. It’s vital to release compressed air while ascending to prevent lung damage.
Ascend slowly
Nitrogen enters the bloodstream during descent compression and is usually released unnoticed over time when normal dive precautions are followed. According to Boyle’s Law, a sudden ascension can create gas bubbles large enough to cause several types of injuries, depending on where the bubbles develop. Safe divers return to the surface at a slow pace to minimize the risk of decompression sickness, and they observe the no-fly rule to give their bodies time to release any accumulated nitrogen before undergoing rapid altitude changes.
How does temperature affect Boyle’s Law?
Volume and pressure have an inverse relationship when temperature is constant. However, if the temperature changes during a descent or ascent, this would affect the amount of volume of the air inside. Although temperature does affect the proportional changes of volume versus pressure, it’s not a huge factor when scuba diving.






